
|
BRHS /
Dispersion in Obstructed EnvironmentWhen studying the hydrogen dispersion in obstructed environment it should be taken into account that the dispersing cloud behaviour completely differs for the cases of gaseous and liquefied hydrogen spills. Actually, hydrogen disperses as a heavier-than-air gas when escapes to the atmosphere from the liquid state and is characterized by horizontal movement and relatively long dilution times, whereas in gaseous form hydrogen is a buoyant gas. In this sense some of the results presented for the dispersion of other flashing liquids could be applicable to the hydrogen dispersion. For example Chan (ChanST:1992) performed calculations for the numerical simulations of LNG vapour dispersion from a fenced storage area, and found that vapour fence can significantly reduce the downwind distance and hazardous area of the flammable vapour clouds. However, a vapour fence could also prolong the cloud persistence time in the source area, thus increasing the potential for ignition and combustion within the vapour fence and the area nearby. Also Sklavounos and Rigas (SklavounosS:2004) performed a validation of turbulence models in heavy gas dispersion over obstacles, which could also be applied for the earlier stages of the spill. In the presence of buildings or other obstacles, the wind direction is also expected to play an important role for the cloud dispersion, due to the shielding effects of these obstacles. The obstacle effect is twofold, in one way it inhibits gas convection, but on the other hand creates turbulence, thereby increasing gas dilution, extending the flammable region, and even accelerating the flame. Hydrogen may cause a series of accidental events (jet fire, flash fire, detonation, fireball, confined vapor cloud explosion), depending on the time of ignition and the space confinement. Unless an immediate ignition occurs, it becomes evident that dispersion calculation is required, in order to determine the lower flammable limit zones in the greater area of hydrogen facilities and hence preventing, via appropriate measures, flash fires and confined vapor cloud explosions corresponding to delayed ignition, see for example the work of Rigas and Sklavounos (RigasF:2005). Though accidents related to storage and use of hydrogen will certainly occur, there is not much data available in the literature about what happens when liquid hydrogen is accidentally released near the ground between buildings of a residential area. Only few numerical codes used for dispersion estimation can be applied to hydrogen, which means that further developmental work is necessary in this field. Statharas et. al. (StatharasJC:2000) describe the modelling of liquid hydrogen release experiments using the ADREA-HF 3-D time dependent finite volume code for cloud dispersion and compare with experiments performed by Batelle Ingenieurtechnik for BAM Bundesanstalt fur Materialforschung und Prufung , Berlin, in the frame of the Euro-Quebec-Hydro-Hydrogen-Pilot-Project. They mainly deal with LH2 near ground releases between buildings. The simulations illustrated the complex behaviour of LH2 dispersion in presence of buildings, characterized by complicated wind patterns, plume back flow near the source, dense gas behaviour at near range and significant buoyant behaviour at the far range. The simulations showed the strong effect of ground heating in the LH2 dispersion, as can be observed comparing figures Fig. 1 and Fig. 2. The model also revealed major features of the dispersion that had to do with the "dense" behaviour of the cold hydrogen and the buoyant behaviour of the "warming-up" gas as well as the interaction of the building and the release wake. 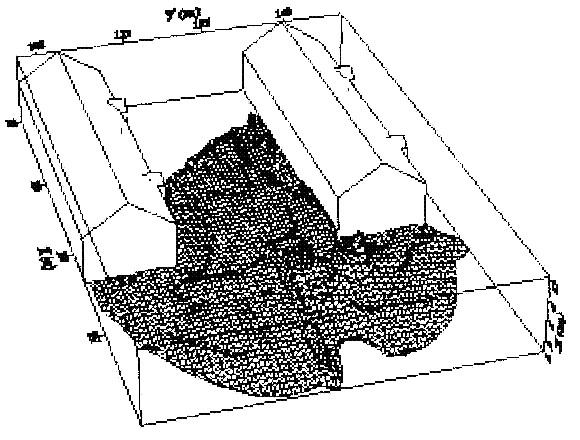 Fig. 1: Predicted 4% iso-surface at t=100 s, without ground heating effects (StatharasJC:2000) 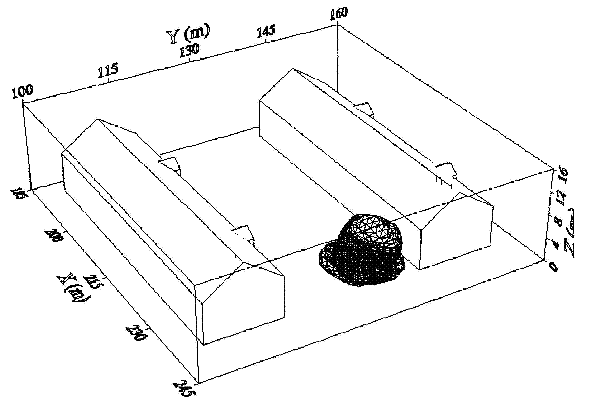 Fig. 2: Predicted 4% iso-surface at t=100 s, with ground heating effects (StatharasJC:2000) Schmidt et. al. (SchmidtD:1999) performed a numerical simulation of hydrogen gas releases between buildings. Gas cloud shape and size were predicted using the Computational Fluid Dynamics Code FLUENT 3. The modelling was made as close as possible to the pattern of the liquid hydrogen release experiments performed by BAM in the framework of the EQHHPP. Four main results were found. The release of hydrogen at high velocities (up to the critical velocity) results in a much more hazardous situation than a release at low exit velocities. At high velocities, high concentrations of H2 near the ground and a considerable enlargement of the range where explosive mixtures occur, have to be expected. See figures Fig. 3 and Fig. 4. 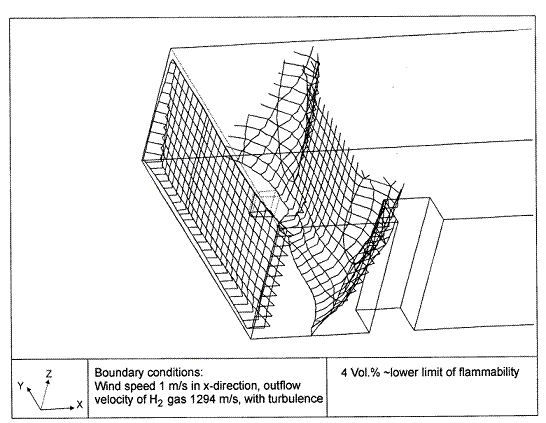 Fig. 3: Fast release in z direction, 4 Vol. % iso-surface (SchmidtD:1999) 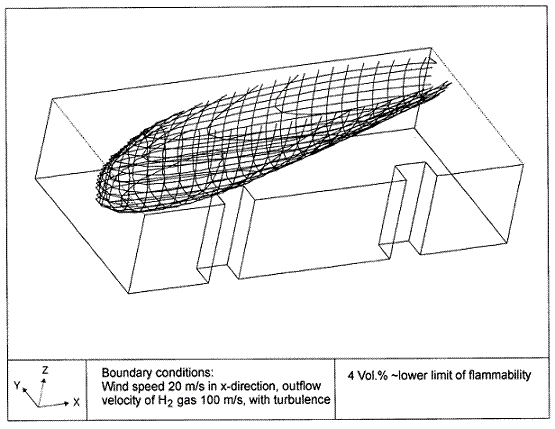 Fig. 4: Slow release in z direction, 4 Vol. % iso-surface (SchmidtD:1999) Venetsanos et. al. (VenetsanosAG:2003) performed a study of the source, dispersion and combustion modelling of an accidental release of hydrogen in an urban environment. The paper illustrates an application of CFD methods to the simulation of an actual hydrogen explosion. Results from the dispersion calculations together with the official accident report were used to identify a possible ignition source and estimate the time at which ignition could have occurred, see Fig. 5. The combustion simulation shows that an initially slow (laminar) flame, that accelerates due to the turbulence generated by the geometrical obstacles in the vicinity (primarily the pressure tanks). Since the hydrogen cloud is very concentrated, with a large region with more than 15% hydrogen by volume, there is ample scope for flame acceleration. However, the general geometrical configuration is rather open, and beyond the bundles of pressure tanks there are few obstacles. This will tend to restrain the acceleration of the flame and prevent the flame from accelerating to very high speeds as seen in the simulations. These results are to a large extent compatible with the reported accident consequences, both in terms of near-field damage to building walls and persons, and in terms of far-field damage to windows. Their results demonstrate that hydrogen explosions in practically unconfined geometries will not necessarily result in fast deflagration or detonation events, even when the hydrogen concentration is in the range where such events could occur in more confined situations 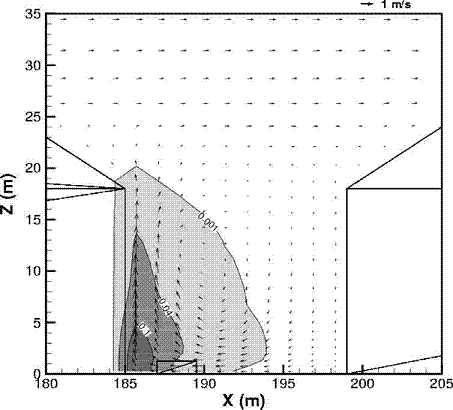 Fig. 5: Predicted velocity and volume concentration field on a vertical cross-canyon plane at 9 m downwind from the source and time 10 s after start of the release (VenetsanosAG:2003) ReferencesInvalid BibTex Entry! |